- 029-86354885
- 18392009562
材料PEOz在sigma的Material Matters上的介紹
西安瑞禧生物科技有限公司近期一直在推廣一種叫聚(2-乙基-2-噁唑啉),PEOz 一種可以替代PEG聚乙二醇的新材料,他比PEG更穩定優點更多,我們公司也一直在尋找資料試圖叫更多客戶認識和體驗這款材料,包括我們推出的PLGA-PEOz和PCL-PEOz的試用活動,就在昨天我們無意中在sigma的官網里面的材料科學-DrugDelivery中的MaterialMatters資料里面的找到了關于介紹PEOz的資料,在SIGMA的資料介紹里確實明確的表述了PEOz作為新的聚合物可以替代PEG衍生物來應用,PEOz比PEG主鏈穩定性更高,活性基團取代率更高,有PH敏感性等特點。
Sigma-Aldrich公司的資料里面主要講述了:
一:PEG雖然作為金牌標準被非常廣泛的應用于基礎生物醫學中,但依然存在“較低的分散度”,“有限的生物相容性”,“有限的生物識別性”等缺點和局限性。例如:在從來沒有使用過PEG藥物的患者體內發現有抗PEG的抗體,這回加速PEG的血液清除。PEG還存在的一個缺點就是PEG的聚醚主鏈易氧化降解。
二:PEOz結構具有更高的穩定性、可調性、較高的生物相容性、更低的分散性、更高的活性基團取代率,具有PH敏感性和做成的二親共聚物有一定的溫敏。
三:PEOz可以做成膠束等材料用于藥學研究
四:其他方面的應用
PEOz具有PH敏感性的原理:
PEOz 中含有叔酰胺基團,該基團及其相應的共振結構容易結合溶液體系中的氫離子,并與PEOz 分子間或分子內的其他叔酰胺基團形成氫鍵,在酸性條件下,大量氫鍵的形成會破壞PEOz 形成膠束的核-殼結構,從而降低膠束的穩定性,釋放藥物。
以下資料引用是Sigma-Aldrich Co. LLC的Material Matters:
POLY(2-OXAZOLINE)S:
THE VERSATILE POLYMERPLATFORM FOR BIOMEDICINE
Introduction
The introduction of polymers into thebiomedical field has opened new avenues in tissue engineering, implant design,biosensing, and drug delivery. The synergetic combination of polymers andpharmaceuticals provides a means to address significant unmet medical needssuch as continuous sustained drug release, or delivery of high drug payloads tospecific tissues. Thus, polymers are a key component in areas such as cancertreatment, regenerative medicine, and gene therapy.
Poly(ethylene glycol), or PEG, also knownas poly(ethylene oxide), or PEO,is the most extensively used polymer inbiomedicine to increase the halflife and immunogenicity of proteins. AlthoughPEG remains the gold standard in polymer-based biomedical applications based onits low dispersity (D), biocompatibility, and limited recognition by the immunesystem (stealth behavior), it has some drawbacks and limitations. For example,anti-PEG antibodies have been observed in a significant number of patients,1,2including 25% of patients never treated with PEG drugs (due to its ubiquity incosmetics and food additives). This suggests the cause of the accelerated bloodclearance of PEG conjugates after multiple injections.3 In addition, thepolyether backbone of PEG is prone to oxidative degradation,4 a significantdrawback for long-term applications such as antifouling surfaces for implants5and probable induction of PEGmediated complement activation.6–8 Nevertheless,the success of PEG in biomedical applications has paved the way for thedevelopment of the next generation of polymeric biomaterials, with greaterversatility and more diverse architectural possibilities to meet the newchallenges in medicine and the requirements in drug loading, responsiveness,targeting and labeling.9–11
Poly(2-alkyl/aryl-2-oxazoline)s, commonlyabbreviated as PAOx, POx,or POZ, provide higher stability, tunability, andfunctionalization than PEG, while retaining the requisite features ofbiocompatibility,12 stealth behavior, and low dispersity. The excellentproperties of PAOx polymers enable their use in a wide variety of differentbiomedical applications,from targeted drug delivery and drug formulation totissue engineering and tissue adhesives. In particular, the extraordinarysynthetic versatility of PAOx allows the construction of complex polymericarchitectures with finely tunable physical properties in a defined manner,making it an attractive platform for developing new approaches in precision medicine.13,14This overview on biomedical applications of PAOx presents a special emphasis ontheir contribution and potential impact on drug delivery applications.
Propertiesand Biocompatibility
As shown in Figure 1, PAOx are readilyobtained via cationic ringopening polymerization (CROP) of 2-oxazolines,resulting in polymers with a backbone composed of tertiary amides that suppressinteractions with proteins and result in significantly reduced recognition bythe immune system.15
Functionalities can be introduced at bothends of the polymer chain by selection of the electrophilic initiator [alkylhalides, acid halides, (pluri) tosylates, (pluri)tri?ates, (pluri)nosylates,etc.] and nucleophilic terminating agent (amines, thiolates, carboxylates,etc.). Control of the polymer chainend functionality allows incorporation oftargeting units or radiolabels for imaging, while also enabling the use of PAOxfor surface or nanoparticle modification. Moreover, the side chains are tunableby modification of the substituent of the 2-oxazoline monomer, granting controlover the hydrophilic–hydrophobic balance and the lower critical solution temperature(LCST) of the polymer.16 This side-chain tunability enables the introduction ofmultiple functional groups along the polymer chain and the preparation ofhydrogels or highly drug-loaded delivery vehicles.
Figure 2 shows a series of PAOx with an increasingdegree of hydrophobicity. While poly(2-methyl-2-oxazoline), or PMeOx, displays ahigher hydrophilicity than PEG,17 PAOx with longer alkylic side-chains exhibitsa thermoresponsive behavior with transition temperatures
progressively lowering with the polymerside-chain hydrophobicity. In contrast to the gold standard thermoresponsivepolymer for biomedical applications, poly(N-isopropylacrylamide) (PNIPAM, LCST= 32 °C, Prod.No. 806471), PAOx exhibits a minimal thermal hysteresis behaviorandthe transition temperature can be fine-tuned by copolymerization of hydrophilicand hydrophobic 2-oxazolines.18 This tunability makes PAOx an ideal polymer forthe development of stimuli-responsive smart materials,with applications indetection, diagnostics and triggered drug delivery.19–21

The structural similarity of PAOx withnatural polypeptides as shown in Figure 2 accounts for their stealth behaviorand excellent biocompatibility. PAOx exhibit a very fast blood clearance andremarkably low uptake in organs of the reticuloendotheliary system, asdemonstrated in biodistribution studies with radio-labeled 5 kDa PMeOx and poly(2-ethyl-2-oxazoline),or PEtOx,22 that show an apparent clearance limit of 40 kDa.23 In vivo toxicityhas shown no adverse effects upon repeated intravenous injections (in rats) of10 and 20 kDa PEtOx in a broad range of concentrations (500 to 2,000 mg/kg).17Perhaps the most reassuring instance of PAOx biocompatibility is thedevelopment of the first commercial PAOx-based pharmaceutical, which iscurrently undergoing first-in-human Phase I clinical trials.24

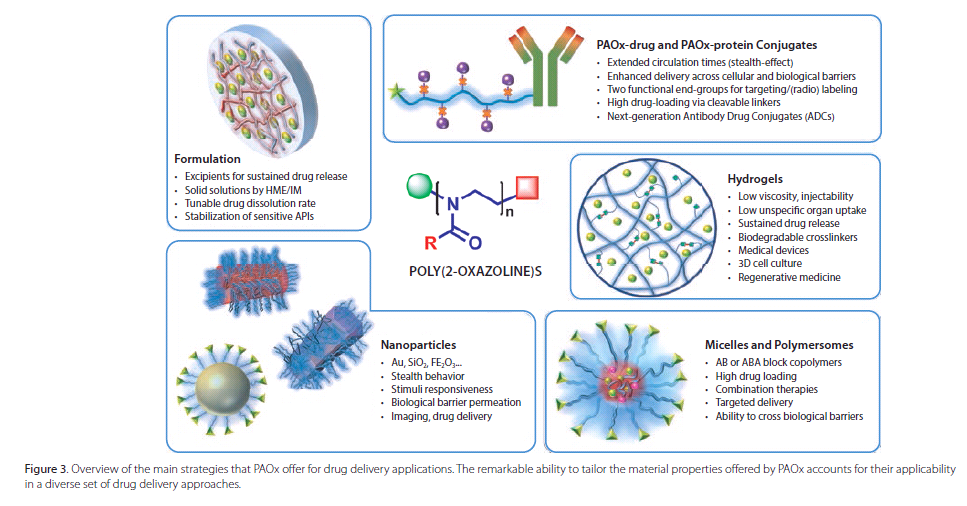
As a result of their excellentbiocompatibility and synthetic versatility,PAOx are attracting a growinginterest as a future platform of choice in drug delivery, and significantprogress in this field has already been realized. Applications in developmenttackle current challenges in high drug loading targeted delivery, combinationtherapy, sustained drug release, and formulation. The main strategiesinvestigated using PAOx in drug delivery are summarized in Figure 3 and can bedifferentiated from systems where the active pharmaceutical ingredient (API) iscovalently or non-covalently linked to the polymer.
Non-covalentlyLinked Drug Delivery
PAOx as an Excipient in DrugFormulation
Perhaps the most straightforwardcontribution of polymers to drug delivery is their use as excipients, where theAPI is dispersed together with the polymer that serves as a matrix to enhancethe drug solubility profile. The search for new drug excipients is motivated bythe poor water solubility properties of an estimated 90% of newly developed drugs.25Hot-melt extrusion (HME) or spray-drying of adequate polymers together with theAPI allows the formation of solid solutions in which the drug is stabilized inan amorphous form, highly increasing its solubility and bioavailability.DeGeest et al. prepared tablet formulations of metoprolol tartrate/PEtOx andfenofibrate/PEtOx via HME using Aquazol. For both the hydrophilic metoprololtartrate and the hydrophobic fenofibrate, release profiles could be eitheraccelerated or slowed down by variation of the polymer molar mass.26 Urbanovaand co-workers demonstrated similar tenability of the acetylsalicylic acidrelease profile in solid dispersions with PEtOx.27In addition, PAOxformulations have been shown to significantly enhancethe stability of sensitivecannabinoids, performing remarkably better than state-of-the-art commercialpolymers.28 Recently developed methods to produce the high molar mass, lowdispersity PAOx29 required for pharmaceutical applications are expected toaccelerate the use of PAOx in drug formulation.
PAOx-based Micelle Systems
Amphiphilic polymers self-assemble intomicelles or polymersomes in which the morphology can be selected by tuning thepolymer length and composition. Micelle systems are advantageous since they canenable high loading of drugs with poor water solubility, a challenge especiallyfor many new cancer treatments. In addition, micelles benefit from both passiveand active targeting because they tend to accumulate in cancer tissues due tothe enhanced permeability and retention (EPR) effect while serving as aplatform to incorporate targeting groups.
PAOx allow for highly defined polymerstructure and composition enabling fine tuning of the hydrophilic–hydrophobicbalance of the polymer by copolymerization and, thus, the control on micellesize and drug release properties. Most reported PAOx-based micellar systems featurea hybrid PAOx-polyester (PAOx-PE) diblock structure, or an ABA triblock structure synthesized bysequential addition of hydrophilic and hydrophobic 2-oxazoline monomers.
Zhao and co-workers used a Boc-NH-PEtOx-OHas a macroinitiator for the polymerization of ε-caprolactone and subsequentlyfunctionalized the hydrophilic PEtOx with a folate moiety. This resulted infolate-decorated micelles that could be loaded with doxorubicin (DOX, Prod. No.D1515) with capacities higher than 10 wt.%. These nanovectors showed better therapeuticefficacy and reduced toxicity than DOX when administered to nude mice bearingKB tumors.30 A similar targeted micelle system was loaded with indocyaninegreen, an FDA-approved near-infrared dye,allowing both tumor imaging as well aseffective photothermal therapy of KB tumors in vivo.31
Multiple targeting moieties can beincorporated in the micelle outer shell for enhanced cellular uptake bycombining differently functionalized PAOx, as recently reported for aPEtOx-b-P(d-l-lactide) system.32
A very well studied micelle systemdeveloped by Kabanov, Jordanand Luxenhofer comprises an ABAtriblock structure featuring a hydrophobic middle-block ofpoly(2-n-butyl-2-oxazoline), or PBuOx, and two outer blocks of PMeOx.33 Thispolymer system yields stable micelles with sizes
below 100 nm and unmatched highdrug-loading capacity of anti-cancer drugs with poor water solubility. Loadingcapacities of up to 50 wt.% have been reported for a range of new-generationtaxoids, increasing the intrinsic solubility of the APIs by up to 9,000times.34 Synergetic effects
arising from combining multiple APIs in themicelles have also been reported for these high capacity micelle systems.35Currently, Kabanov’s team is building a cheminformatic database to predictwhich drugs can best take advantage of these PAOx-based micelle carriers.
PAOx-based Hydrogels
The introduction of functionality across the polymer side-chain allowsa wide variety of strategies to prepare PAOx-based hydrogels.36,37Lecommandoux et al. introduced reactive amine units along the PEtOxchain by partial hydrolysis. The obtained PEtOx–PEI copolymers weresubsequently crosslinked with a bis-glycidyl ether in aqueous medium,resulting in biocompatible spherical nanogels with an optimal size fordrug delivery applications.38,39 Furthermore, injectable hydrogels basedon PEtOx-poly(ε-caprolactone)-PEtOx have demonstrated superiorbiocompatibility compared to commercial Matrigeland PluronicF127(Prod. No. P2443) for intraocular drug delivery in vivo.40
Dargaville et al. copolymerized MeOx and2-(dec-9-enyl)-2-oxazoline, obtaining hydrophilic polymers with alkeneside-chains that were functionalized with the CRGDSG peptide sequence topromote cell adhesion. Subsequent crosslinking in the presence of a dithiolyielded transparent hydrogels in a one-pot fashion. The mild conditions of the gelationprocess permitted the encapsulation of fibroblast cells during the UV-mediatedcuring process, obtaining three-dimensional cell-polymer constructs of interestin tissue engineering and regenerative medicine
CovalentlyLinked Drug Conjugates
PAOx-drug and PAOx-protein Conjugation orPAOxylation
The PAOxylationof a number of proteins like trypsin, catalase, serum albumin, insulin, oruricase has yielded conjugates with performances similar to their PEGylatedcounterparts.42,43 Interestingly, PEtOx-insulin conjugates were found todecrease blood glucose levels for up to 8 hours,four times longer than the freeinsulin.17 Kabanov et al. functionalized a number of piperazine-terminated PAOxwith an NHS-activated ester andprepared conjugates of horseradish peroxidase.44 Theprotein retained 90% of its activity, and the cellular uptake was found toincrease by three to six fold compared to unmodified protein when using anamphiphilic PMeOx- or PEtOx-b-PBuOx copolymer. Similar copolymers were used to conjugatesuperoxide dismutase 1 (SOD1), showing enhanced neuronal uptake of theconjugate in vitro and effective passage through the bloodbrain barrier in vivo
Theintroduction of clickable groups along the hydrophilic PMeOx or PEtOx chain46has proven to be an effective strategy for protein and drug conjugation.Copolymers of MeOx and EtOx with 2-(pent-4-ynyl)-2-oxazoline, or PynOx,provided multiple linkage points for the stabilization of virus-like particles(VLP). An icosahedral VLP was formed by supramolecular assembly of 180 copiesof the coat protein of bacteriophage Qβ, and its surface was decorated withazide groups
using anazido-N-hydroxysuccinimide ester. Following copper-catalyzed azide-alkynecycloaddition (CuAAC) click with PMeOx/PEtOx-ran-PynOx copolymers resulted inPAOx-wrapped VLPs with remarkably high thermal stability. Furthermore, theparticle size could be controlled by the polymer length and attachment density.
SerinaTherapeutics has used similar PEtOx-ran-PynOx polymers to create a one-weeklong sustained release of rotigotine for the treatment of Parkinson’s disease.The API is linked to the polymer via CuAAC click chemistry using abiodegradable ester spacer, enabling sustained drug
release thatleads to constant plasma levels.48 This polymer, named SER?214, iscurrently undergoing Phase I clinical trials and, if successful, will becomethe first FDA-approved PAOx therapeutic.
Hoogenboom etal. introduced methyl ester functionalities across the polyoxazoline chain bycopolymerization of EtOx with 2-methoxycarbonylethyl-2-oxazoline (MestOx).49The authors
demonstratedthat the resulting methyl ester functionalities decorating the polyoxazolinechain constitute a highly versatile reactive handle, as a wide variety ofmoieties can be introduced by direct amidation with amines. This syntheticapproach further expands the PAOx toolbox for the
preparation ofnovel PAOx-drug conjugates
Future multipledrug-loaded PAOx-API conjugates will most definitely be improved by theaddition of targeting moieties in the polymer chainends, such as folate groupsor antibodies. There is, thus, ample room for advances in this fascinatingfield.
PAOx-functionalized Nanoparticles
Nanoparticles(NPs) are able to accommodate multiple functional groups while providing uniqueoptical, electronic, or magnetic properties and, therefore, have enormouspotential in biomedical sciences, including imaging and drug delivery. Whenconnected to NPs, PAOx form a stealth
corona thatprovides nanoparticle stabilization, prevents rapid clearance,and serves as areliable scaffold for the incorporation of bioactive compounds. In thiscontext, Benetti and co-workers functionalized PMeOx-OH with nitrodopamine forthe functionalization of ZnO nanocrystals of interest for imaging.Functionalization with 4 kDa PMeOx yielded individually dispersed nanocrystalswith an outstanding stability of up to 9 months
In addition togranting stability and stealth effect to NPs, PAOx thermoresponsive propertieshave been exploited to prepare responsive or smart NPs that aggregate upon theapplication of external stimuli.20 Recently, fluorescent NPs based on apolyorganosiloxane were functionalized with the thermo-responsivepoly(2-isopropyl-2-oxazoline), or PiPrOx, (Figure 2).Below 31 °C, the PiPrOxnanoparticles exhibit an anti-fouling behavior when dispersed in aserum-containing medium. However, heating beyond this temperature triggers theadsorption of serum proteins on the
nanoparticles,reversible by lowering the temperature. This strategy could be applied to increasenanoparticle agglomeration in the targeted cells or organs by applying localheating
Finally, asseen before for PAOx-based micelles and conjugates, PAOx can enhancenanoparticle permeation through biological barriers.Khutoryanskiy et al. used 5kDa alkyne-terminated PEtOx to functionalize thiolated silica NPs via thiol-enechemistry. The resulting NPs exhibited enhanced permeation through porcinegastric mucosa ex vivo in a similar way as analogous PEGylated NPs.52Considering the straightforward tunability of PAOx composition andhydrophilic–hydrophobic balance, further developments are expected to bringeven more efficient drug delivery vehicles with improved ability to permeatebiological barriers
西安瑞禧生物科技有限公司可以提供非常廣泛的PEOz產品:
BlockCopolymers PEOz
mPEOz-PCL
mPEOz-PLGA
mPEOz-PLA
PCL-PEOz-MAL
PCL-PEOz-NHS
PCL-PEOz-NH2
PCL-PEOz-COOH
PCL-PEOz-Biotin
PCL-PEOz-Folate
PCL-PEOz-cRGD
PCL-PEOz-FITC
PLGA-PEOz-NHS
PLGA-PEOz-MAL
PLGA-PEOz-COOH
PLGA-PEOz-NH2
PLGA-PEOz-Folate
PLGA-PEOz-FITC
PLGA-PEOz-OH
PLA-PEOz-NHS
PLA-PEOz-MAL
PLA-PEOz-COOH
PLA-PEOz-NH2
PLA-PEOz-Folate
PLA-PEOz-FITC
PLA-PEOz-OH
PEOzylation, Lipids(PEOz-PE) PEOz
DSPE-PEOz
DPPE-PEOz
DSPE-PEOz-NHS
DSPE-PEOz-NH2
DSPE-PEOz-COOH
DSPE-PEOz-MAL
DSPE-PEOz-Biotin
DSPE-PEOz-FA
DSPE-PEOz-cRGD
DSPE-PEOz-FITC
Amine(NH2) PEOz
mPEOz-NH2
NH2-PEOz-NH2
NH2-PEOz-COOH
NH2-PEOz-SH
NH2-PEOz-Alkyne
NH2-PEOz-FA
NH2-PEOz-MAL
SuccinimidylCarboxymethyl(NHS)PEOz
mPEOz-NHS
NHS-PEOz-NHS
NHS-PEOz-COOH
NHS-PEOz-OH
NHS-PEOz-MAL
Acryloyl-PEOz-NHS
CarboxylicAcids (COOH) PEOz
mPEOz-COOH
COOH-PEOz-COOH
MAL-PEOz-COOH
Acryloyl-PEOz-COOH
Alkyne-PEOz-COOH
Biotin-PEOz-COOH
Biotin(Bio) PEOz
mPEOz-Biotin
Biotin-PEOz-Biotin
Biotin-PEOz-NHS
Biotin-PEOz-MAL
Biotin-PEOz-NH2
Biotin-PEOz-OH
Biotin-PEOz-Alkyne
Maleimides(MAL) PEOz
mPEOz-MAL
MAL-PEOz-MAL
MAL-PEOz-OH
MAL-PEOz-NH2
MAL-PEOz-Alkyne
MAL-PEOz-OPSS
Thiols(SH) PEOz
mPEOz-SH
HS-PEOz-SH
HS-PEOz-COOH
Alkyne-PEOz-SH
HS-PEOz-NHS
HS-PEOz-Biotin
HS-PEOz-Silane
Silane(SIL) PEOz
mPEOz-Silane
Silane-PEOz-Silane
Silane-PEOz-Biotin
Silane-PEOz-NHS
Silane-PEOz-COOH
NH2-PEOz-Silane
MAL-PEOz-Silane
HO-PEOz-Silane
Alkyne-PEOz-Silane
Hydroxyls(OH) PEOz
HO-PEOz-COOH
HO-PEOz-NHS
HO-PEOz-NH2
HO-PEOz-Alkyne
HO-PEOz-SH
ProtectedAmines (FMOC, tBOC) PEOz
BOC-NH-PEOz-OH
FMOC-NH-PEOz-OH
BOC-NH -PEOz-NH2
FMOC-NH-PEOz-NH2
BOC-NH -PEOz-COOH
FMOC-NH-PEOz-COOH
BOC-NH -PEOz-NHS
FMOC-NH-PEOz-NHS
BOC-NH-PEOz-MAL
FMOC-NH-PEOz-MAL
BOC-NH-PEOz-SH
FMOC-NH-PEOz-SH
OrthopyridylDisulfide (OPSS) PEOz
OPSS-PEOz-NHS
OPSS-PEOz-OPSS
OPSS-PEOz-NH2
OPSS-PEOz-COOH
OPSS-PEOz-Biotin
OPSS-PEOz-OH
Fluorescent PEOz
mPEOz-FITC
FITC-PEOz-NHS
FITC-PEOz-MAL
FITC-PEOz-NH2
FITC-PEOz-SH
FITC-PEOz-COOH
FITC-PEOz-Biotin
FITC-PEOz-N3
FITC-PEOz-Silane
FITC-PEOz-Alkyne
FITC-PEOz-OH
mPEOz-RB
RB-PEOz-OH
RB-PEOz-NH2
RB-PEOz-COOH
RB-PEOz-MAL
CY3-PEOz-NH2
CY3-PEOz-COOH
CY3-PEOz-NHS
CY3-PEOz-MAL
CY3-PEOz-SH
CY3-PEOz-Biotin
CY5-PEOz-NH2
CY5-PEOz-COOH
CY5-PEOz-NHS
CY5-PEOz-MAL
CY5-PEOz-SH
CY5-PEOz-Biotin
CY7-PEOz-NH2
CY7-PEOz-COOH
CY7-PEOz-NHS
CY7-PEOz-MAL
CY7-PEOz-SH
CY7-PEOz-Biotin
含親水段聚合物PEOz的二親共聚物:
我們可以提供PEOz和PEI、殼聚糖、聚賴氨酸PLL,聚谷氨酸,聚天冬氨酸,葡聚糖,透明質酸,PS聚苯乙烯等等形成二親共聚物用于藥物載體的制備
西安瑞禧生物科技有限公司可以提供以下材料:
PEOz-PEI
PEOz-Chitosan
PEOz-PLL
PEOz-PGA 溫度和pH值雙敏感
PEOz-PASP
PEOz-Dextran
PEOz-HA
PEOz-PS
PEOz-PMMA
PEOz-PNIPAM
| 序號 | 新聞標題 | 瀏覽次數 | 作者 | 發布時間 |
|---|---|---|---|---|
| 1 | 瑞禧定制-功能化1,2,4,5-四嗪Cis-[Pt-1,3-Propanediamine]-2-Me-Tetrazine/IC-MethylTetrazine | 800 | 瑞禧生物 | 2022-11-09 |
| 2 | 科研-四嗪Py-Tetrazine-PEG1-Alkyne/Py-PEG1-Alkyne/Pyrimidine-Tetrazine-PEG1-Alkyne | 826 | 瑞禧生物 | 2022-11-09 |
| 3 | 胺基與NHS活性酯反應PEG之Azido-PEG7-amine/1333154-77-0瑞禧生物 | 1591 | 瑞禧生物 | 2023-01-03 |
| 4 | 瑞禧2023更新 Azido-PEG8-acid疊氮八聚乙二醇羧酸 | 727 | 瑞禧生物 | 2023-01-03 |
| 5 | 嵌段共聚物4 arm-PEG-TK-NH2 /NHS/MAL | 830 | 瑞禧生物 | 2022-12-08 |
| 6 | 活性氧敏感聚合物TK-PPE 酮縮硫醇-聚磷酸酯 PPE-TK | 877 | 瑞禧生物 | 2022-12-08 |
| 7 | 功能化腙鍵響應性磷脂 DSPE-Hyd-PEG-Alkyne/CHO/cRGD 醛基/多肽 | 859 | 瑞禧生物 | 2022-12-08 |


 400-115-0588
400-115-0588 在線咨詢
在線咨詢







 庫存查詢
庫存查詢